!!UPDATE MAR 31, 2025 – The LDT Final Rule was overturned in court!!
Get more info from ACLA
———————- From October 2024 ——————————-
Summary of the issue:
FDA announced new actions to strengthen the safety and effectiveness of Laboratory Developed Tests (LDTs) through stricter regulations and targeted enforcement.
- Increased FDA Oversight: The FDA is actively working to ensure the safety and effectiveness of Laboratory Developed Tests (LDTs) through targeted enforcement and new regulations.
- Focus on Patient Safety: The primary goal of the FDA’s actions is to protect patients and healthcare professionals by ensuring LDTs are accurate and reliable.
- Continued Access to Critical Tests: While implementing stricter regulations, the FDA aims to maintain patient access to crucial LDTs needed for various health care decisions.
“LDTs” are now termed “IVDs offered as LDTs” and they will be regulated as medical devices by FDA within the framework of 21 CFR 820 Quality System Regulation
The Final Rule:
Federal Register :: Medical Devices; Laboratory Developed Tests
Federal Courty Vacates FDA Rule (update)
Timeline (updated):
October 3, 2023 – FDA Issues Proposed Rule on LDTs
-
The FDA published a proposed rule to regulate LDTs as medical devices under the Federal Food, Drug, and Cosmetic Act (FDCA), aiming to phase out its longstanding policy of enforcement discretion.
April 29, 2024 – FDA Announces Final Rule
-
The FDA announced the finalization of the rule, maintaining the core provisions of the proposed rule.
May 6, 2024 – Final Rule Published in Federal Register
-
The final rule was officially published in the Federal Register, amending the definition of in vitro diagnostic products to include those manufactured by laboratories.
July 5, 2024 – Final Rule Becomes Effective
-
The final rule took effect 60 days after publication.
August 19, 2024 – Association for Molecular Pathology Files Lawsuit
-
The Association for Molecular Pathology (AMP) filed a lawsuit in the U.S. District Court for the Southern District of Texas, challenging the FDA’s authority to regulate LDTs as medical devices.
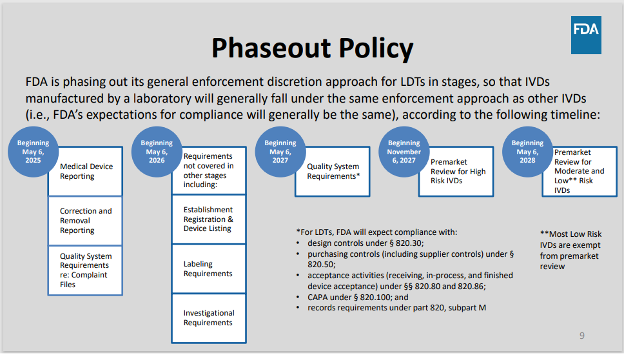
May 6, 2025 – Stage 1 of FDA’s Phaseout Policy Scheduled to Begin
-
Under the final rule, this date marked the beginning of Stage 1, requiring compliance with medical device reporting, correction and removal reporting, and certain quality system requirements.
March 31, 2025 – Federal Court Vacates FDA’s Final Rule
-
The U.S. District Court for the Eastern District of Texas vacated the FDA’s final rule, ruling that the agency exceeded its statutory authority by classifying LDTs as medical devices.
The historical information is shown below for reference, but is no longer in effect:
- Stage 1: beginning on May 6, 2025, which is 1 year after the publication date of the final LDT rule, FDA will expect compliance with medical device reporting (MDR) requirements, correction and removal reporting requirements, and quality system (QS) requirements regarding complaint files.
- Stage 2: beginning on May 6, 2026, which is 2 years after the publication date of the final LDT rule, FDA will expect compliance with requirements not covered during other stages of the phaseout policy, including registration and listing requirements, labeling requirements, and investigational use requirements.
- Stage 3: beginning on May 6, 2027, which is 3 years after the publication date of the final LDT rule, FDA will expect compliance with QS requirements (other than requirements regarding complaint files which are already addressed in stage 1).
- Stage 4: beginning on November 6, 2027, which is 3½ years after the publication date of the final LDT rule, FDA will expect compliance with premarket review requirements for high-risk IVDs offered as LDTs (IVDs that may be classified into class III or that are subject to licensure under section 351 of the Public Health Service Act), unless a premarket submission has been received by the beginning of this stage in which case FDA intends to continue to exercise enforcement discretion for the pendency of its review.
- Stage 5: beginning on May 6, 2028, which is 4 years after the publication date of the final LDT rule, FDA will expect compliance with premarket review requirements for moderate-risk and low-risk IVDs offered as LDTs (that require premarket submissions), unless a premarket submission has been received by the beginning of this stage in which case FDA intends to continue to exercise enforcement discretion for the pendency of its review.
FDA Webinars: (source – CDRH Learn | FDA)
Enforcement Policies for Certain In Vitro Diagnostic Devices – Draft Guidances (Updated 6/11/2024)
Presentation External Link Disclaimer Printable Slides Transcript
In Vitro Diagnostic Product (IVD): Classification (Updated 8/18/2024)
Presentation External Link Disclaimer Printable Slides Transcript
In Vitro Diagnostic Products (IVDs) – MDR Requirements, Correction and Removal Reporting Requirements, and Quality System Complaint Requirements (Updated 8/27/2024)
Presentation External Link Disclaimer Printable Slides Transcript
Labeling Requirements for In Vitro Diagnostic Products (IVD), Including LDTs, Under 21 CFR 809.10(b) (Updated 9/27/2024)
Presentation External Link Disclaimer Printable Slides Transcript
Laboratory Developed Tests; Medical Devices: Proposed Rule
PresentationExternal Link Disclaimer Printable Slides Transcript
Exclusions: