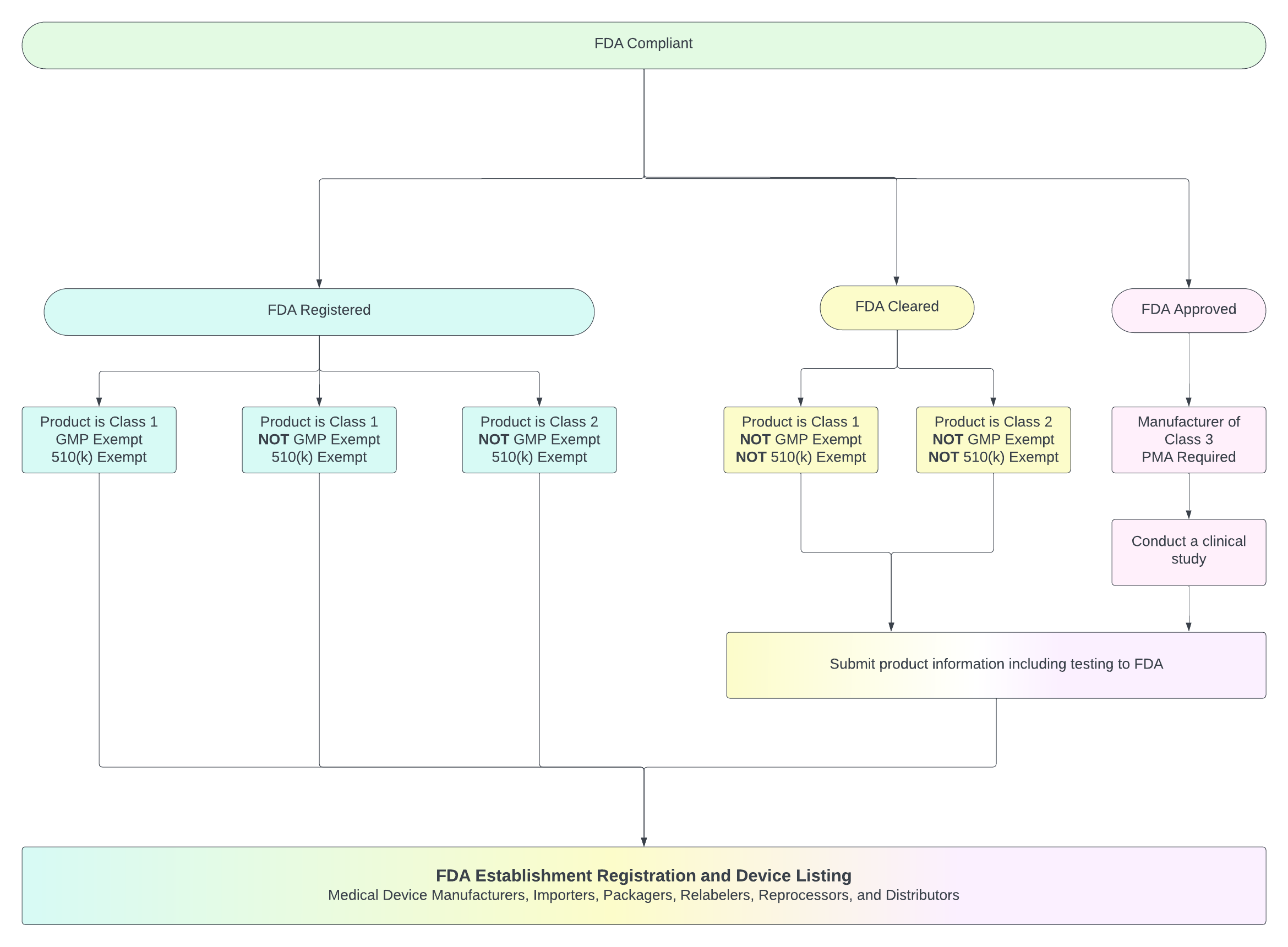
Beyond the 510(k): Why “GMP-Exempt” Status Matters in Medical Device Classification
Navigating the world of medical device regulation can feel like deciphering a complex code. You’re likely familiar with device classes (Class I, II, and III) and submission types like the Premarket Notification 510(k) or Premarket Approval (PMA). These details are undeniably important in determining the path to market clearance or approval. However, when examining a device’s classification details, there’s another requirement that often gets overlooked: the field labeled “GMP Exempt?” in the Product Classification database. This often-overlooked status can affect everything from your documentation burden to inspection readiness.
What is GMP?
GMP stands for Good Manufacturing Practices, and for medical devices in the United States, these requirements are primarily set forth in the FDA’s Quality System (QS) Regulation, codified in 21 CFR Part 820. This regulation acts as an “umbrella” framework designed to ensure that medical devices are safe and effective and consistently meet applicable requirements and specifications.
The QS regulation is comprehensive, covering essential elements of a quality system. These include key elements like design controls (particularly for Class II and III devices, and some Class I), document control, purchasing controls, device identification and traceability, production and process controls, acceptance activities, handling of non-conforming products, corrective and preventive actions (CAPA), labeling and packaging control, records (including complaint files and device history records), and more. Adhering to these requirements is critical; non-compliance can potentially lead to regulatory actions such as warning letters or plant shutdowns.
The QS regulation provides a framework that manufacturers must follow, requiring them to develop and follow procedures appropriate to their specific devices and operations. While the regulation sets the essential elements, manufacturers have flexibility to tailor the specific procedures to their particular processes and devices.
The “GMP Exempt” Status
The FDA has determined that certain types of medical devices are exempt from the full GMP requirements. These exemptions are specified in the FDA classification regulations found in 21 CFR 862 to 892. This is where the “GMP Exempt?” field comes in. When a device classification indicates “GMP Exempt? Yes,” it means that particular type of device is relieved from many of the detailed requirements outlined in 21 CFR Part 820. If it says “GMP Exempt? No,” the manufacturer must comply with the full QS regulation requirements applicable to their device.
Why This Status is So Important
Understanding the GMP Exempt status is crucial because it directly dictates the extent of the quality system controls and manufacturing procedures a manufacturer must implement and maintain.
Consider these examples:
-
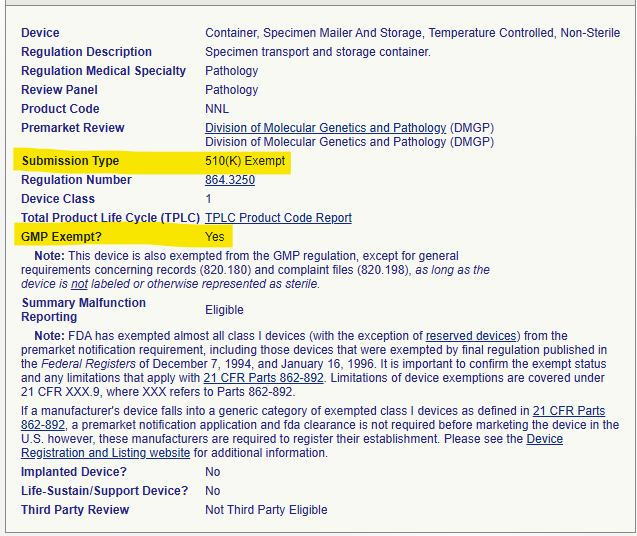
- A Class 1 device, like the Specimen Mailer and Storage Container (Product Code NNL), is 510(k) Exempt. This means it doesn’t require a premarket notification submission and is listed as “GMP Exempt? Yes”. This indicates this product is exempt from the main QS regulation requirements.
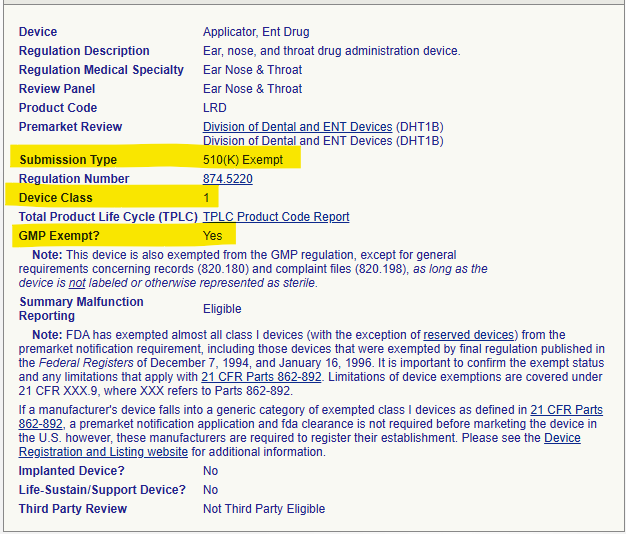
- However, another Class 1 device, such as the Applicator, ENT Drug (Product Code LRD), is 510(k) exempt, but is listed as “GMP Exempt? No”. Despite not needing a 510(k) submission, the manufacturer of this kit must comply with the full applicable Quality System Regulation.
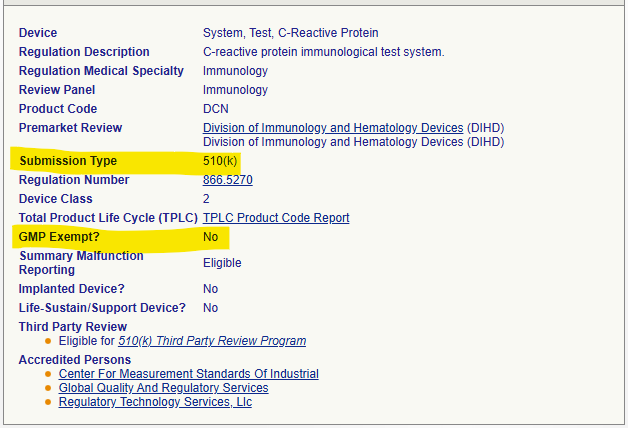
- Moving to Class 2, a device like the C-Reactive Protein test system (Product Code DNC) requires a 510(k) submission (“Submission Type: 510(k)”). It is also listed as “GMP Exempt? No”, confirming the manufacturer must comply with the applicable QS regulation requirements.
These examples highlight that 510(k) exemption status and GMP exemption status are distinct regulatory considerations. A device can be 510(k) exempt but not GMP exempt, or it can be both 510(k) exempt and GMP exempt. And this distinction greatly impacts how your company operates and the type and level of documentation you must maintain.
Important Caveats for GMP Exempt Devices
It is vital to note that even when a device is determined to be GMP exempt, the exemption does not relieve manufacturers from all regulatory requirements. Specifically, manufacturers of finished devices that are GMP exempt are generally still required to keep complaint files (21 CFR 820.198) and comply with general requirements concerning records (21 CFR 820.180). The specific classification regulation for the device type should always be consulted to confirm which requirements apply and any limitations to the exemption.
In Conclusion
While the product code and submission type (510(k) or exempt) provide essential information about a medical device’s regulatory pathway, the “GMP Exempt?” field adds another critical layer of understanding. It tells you whether the extensive Quality System (GMP) requirements of 21 CFR Part 820 apply to the device type or if it is exempt from many of those controls. Knowing this status is fundamental for manufacturers to establish and maintain the appropriate quality system, ensuring their devices are not only cleared or approved for market but are also manufactured in a way that provides reasonable assurance of their safety and effectiveness. Always check the Product Classification database and relevant regulations to confirm the specific requirements for any medical device.